
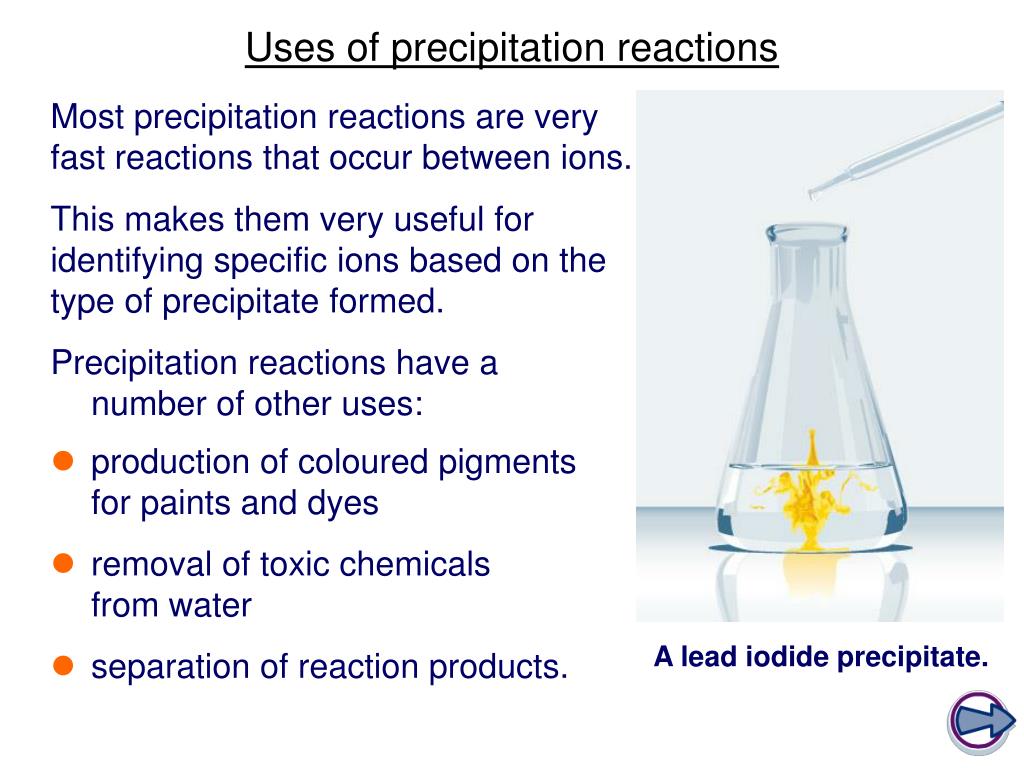
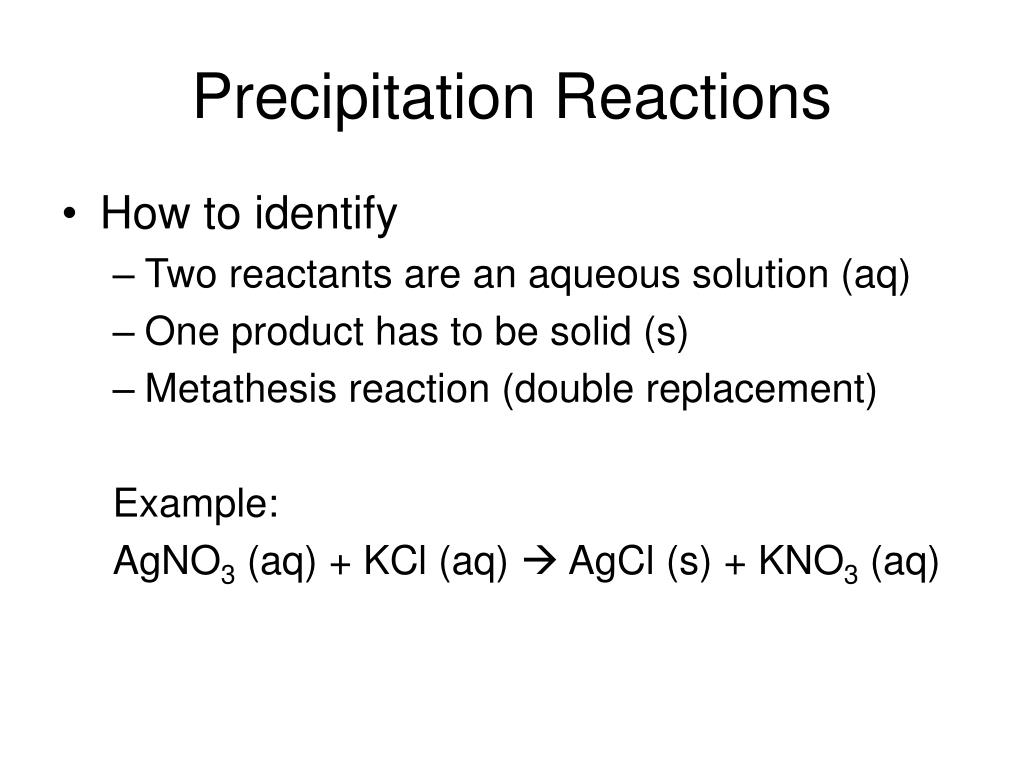
The chemical reaction between potassium chloride (KCl) and silver nitrate (AgNO 3), and solid silver chloride (AgCl) is the precipitate or the insoluble salt formed as a product of the reaction is one of the examples of a precipitation reaction. Thus, either its cation or anion starts bonding with another ion in solution. In a single replacement reaction, one ionic compound dissociates. In a double displacement reaction, both ionic reactants dissociate in water and their ions bonds with the respective cation or anion from the other reactant.įor a double displacement reaction to be a precipitation reaction, one of the resulting products must be insoluble in aqueous solution. They can be single displacement reactions or double displacement reactions. These insoluble salts formed are precipitates that are the products of it. It means the chemical reaction occurs in aqueous solutions where two ions bond together to form insoluble salts. Thus, the reactants can be in forms of solid, gas or liquid.
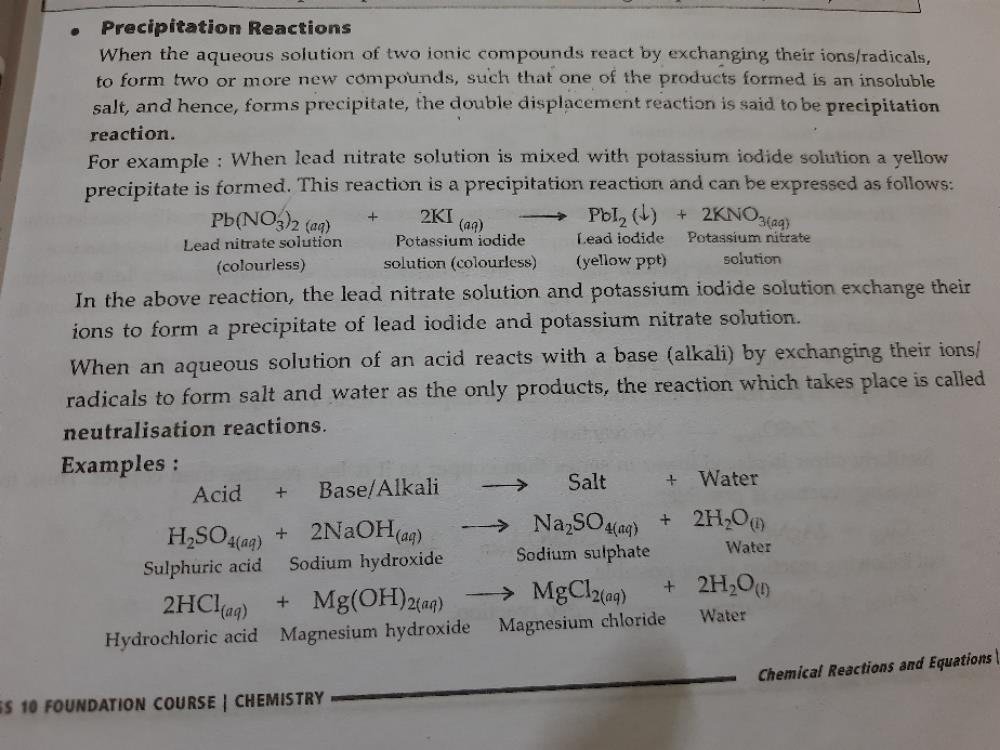
Moreover, chemical reactions occur between two or more chemical compounds that we refer to as reactants. Thus, it gives rise to a new element under some particular conditions. Further, chemical reactions consist of chemical changes that take place within the substances. 1.6 Solved Question for You Precipitation ReactionĪ precipitation reaction is a chemical reaction that occurs in aqueous solution and form precipitates.


 0 kommentar(er)
0 kommentar(er)
